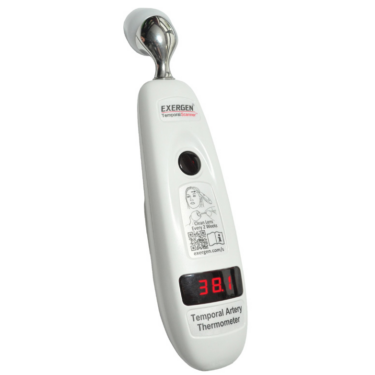
Clever IR Adds the MSV to its AutoSmart IRT/C Product Line Focused on Cleverly Enhancing Accuracy
CleverIR (formerly named Exergen Global) announced the MSV edition as its latest addition to its extensive AutoSmart IRt/c family line which already consists of a broad family line of 11 AutoSmart IRt/c sensors. The AutoSmart Transmitter is unique as it is the first transmitter in the world capable of fully…